
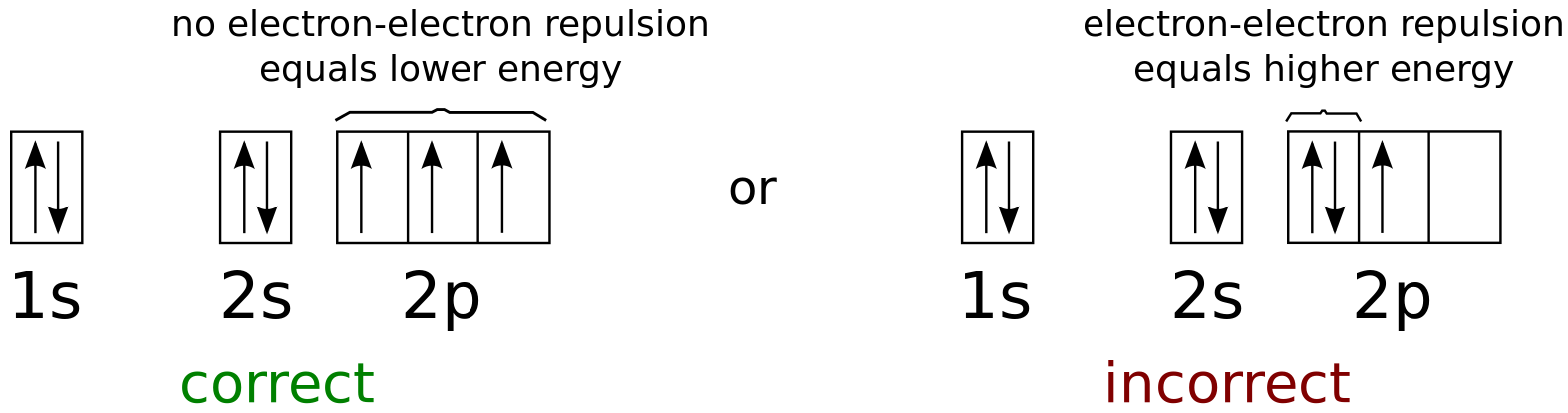

It means that its first subshell of a shell filled with 2, second s subshell of a shell filled with 2 and 2 nd p subshell of shell filled with 3 electrons. To demonstrate the management of electrons in the subshells of atoms of an element C, P, D, F uses the notation.Ī number of electrons in a subshell is demonstrating as a given example:įor example Electronic configuration of Nitrogen (Atomic number = 7) is 1s 2, 2s 2, 2p 3. The arrangement of an electron in the subshell of an element’s atoms is called the electron configuration of that element. This sequence continues until all the electrons of the atom enter the subshell. After fill, 2s subshell electrons enter into 2p subshell. So after 2 electrons in a 1s subshell, electrons enter the 2s subshell. The maximum number of electrons in s, p, d, and f subshells Subshells, it means the stability of the 1s subshell is the highest and theĮlectron will first enter into this subshell. The energy of the 1s subshell is the lowest among all the This is the easy way of remembering the increasing order of energies of the atoms’ subshells.
#Aufbau principle order serial number
It is also called the serial number of shells or shells of atoms. The number before s, p, d, and f word is the main quantum number of electrons of its subshells. The following is the increasing order of energies of the Aufbau PrincipleĮlectrons enter the subshells of atoms in the increasing order of energy. The electrons in the subshell of atoms fill according to the Aufbau principle. Electron Configuration: Aufbau is a word of german language.


 0 kommentar(er)
0 kommentar(er)
